Last Updated on September 25, 2023
This page describes the ecology of JEV, including features of the vertebrate hosts and mosquito vectors in Asia and Oceania, and potentially competent mosquito vectors and host species (mammals, birds and bats) in North America.
JEV life cycle
In nature, Japanese encephalitis virus (JEV) ecology is maintained in a complex transmission cycle that includes vertebrate maintenance hosts (particularly birds), vertebrate amplifying hosts (including both birds and pigs), and mosquito vectors.
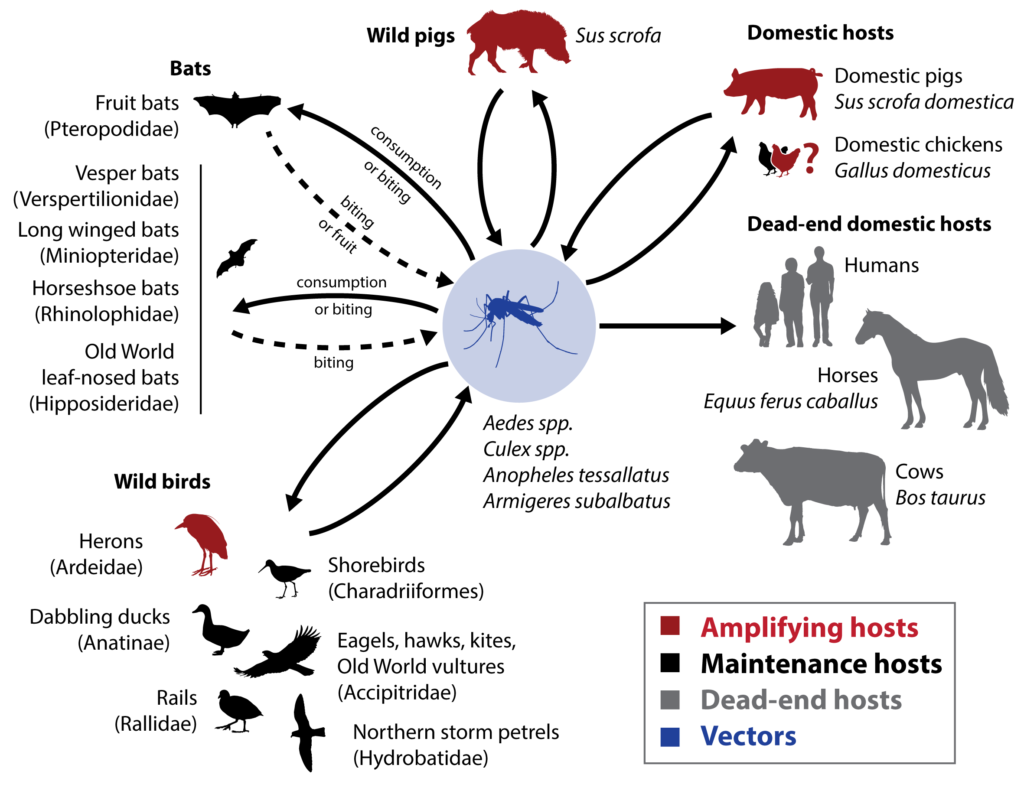
Japanese encephalitis virus (JEV) transmission cycle. The diagram includes clades known to host JEV as documented via samples collected from the wild. The status of domestic chickens as amplifying hosts is suggested by models (Walsh et al. 2022). Image credit: Éric Marty, CEID, University of Georgia.
JEV dead-end hosts
JEV dead-end hosts are vertebrate species that can be infected with JEV but do not develop sufficient concentrations of virus particles (i.e. viremia) to infect biting mosquitoes. Cattle, horses, humans, water buffalo, and small domestic and small wild mammals are dead-end hosts (Rosen, 1986 and Van den Eynde et al., 2022). Although JEV has been isolated from captured snakes and frogs in Korea and a captured gecko in Japan, additional research is needed to understand if naturally infected amphibians, reptiles, and other vertebrate species develop sufficient viremia to infect biting mosquitoes (Chang, 1961, Lee, 1968, and Rosen, 1986).
JEV maintenance hosts
Birds
Known JEV maintenance hosts include wading birds in the Ardeidae family such as the Black-crowned night heron (Nycticorax nycticorax), Cattle egret (Bubulcus ibis), Great egret (Adrea alba), Little egret (Egretta garzetta), and Plumed egret (Egretta intermedia). JEV infections in Ardeid birds are typically asymptomatic.
JEV antibodies have also been collected from other wild bird species. According to Saito et al. (2009), on Hokkaido Island, Japan, over 80% of captured Indian Spot-billed ducks (Anas poecilorhyncha), Mallard ducks (Anas platyrhynchos), and Northern pintails (Anas acuta) and over 30% of captured Eurasian wigeons (Mareca penelope) had JEV-specific antibodies, suggesting that wild ducks might play an important role as a JEV reservoir. In a serosurveillance study of wild birds in Korea, JEV antibodies were found in Band-rumped Storm petrels (Oceanodroma castro), Baikal teals (Sibirionetta formosa), Eurasian coots (Fulica atra), Eurasian teals (Anas crecca), Eurasian wigeons (Mareca penelope), Indian Spot-billed ducks (Anas poecilorhyncha), Northern pintails (Anas acuta), Mallard ducks (Anas platyrhynchos), and Mandarin ducks (Aix galericulata), with at least 78% of samples of each species testing positive for JEV antibodies (Yang et al., 2011).
In Singapore, captured Common redshanks (Tringa tetanus), Lesser sandplover (Charadrius mongolus), and Pacific golden plovers (Pluvialis fulva), which all migrate along the East-Asia Australian Flyway (EAAF), showed a 9.45% positivity rate for JEV antibodies (Yap et at., 2019). Wild-resident birds in Singapore with JEV antibodies include the Changeable Hawk-eagle (Nisaetus cirrhatus), Crested Goshawk (Accipiter trivirgatus), Crested Serpent-Eagle (Spilornis cheela), Oriental Honey-buzzard (Pernis ptilorhynchus), and White-bellied sea eagle (Haliaeetus leucogaster).
In North America, common grackles (Quiscalus quiscula), European starlings (Sturnus vulgaris), house finches (Haemorhous mexicanus), House sparrows (Passer domesticus), Mallard ducks (Anas platyrhynchos), Red-winged blackbirds (Agelaius phoeniceus), Ring-billed gulls (Larus delawarensis), and Rock pigeons (Columbia livia) experimentally inoculated with JEV all had detectable, albeit moderate JEV titers of 101.7 – 5.4 plaque-forming units (PFU/mL) (Nemeth et al., 2012). Although other avian species in the same experiment, such as the American crow (Corvus brachyrhynchos), did not seroconvert above the minimum threshold of 101.7 PFU/ml, the results of the experiment demonstrated that birds may have an important role influencing JEV epidemiology in North America.
Domestic chickens and ducks are susceptible to asymptomatic JEV infection and may be JEV maintenance hosts. In Indonesia, a JEV seroprevalence survey conducted in 9 administrative districts in Bali Province determined that backyard domestic chickens and ducks raised with domestic pigs had average JEV seroprevalence of 36.7% and 20.7 %, respectively (Adi et al., 2016). Pigs had an average JEV seropositivity rate of 32.2%. The same study also determined that in one village, JEV seroprevalence in ducks raised with pigs was 52% compared to JEV seroprevalence of 55.1% in ducks that were raised in the absence of pigs in another village. JEV seroprevalence between duck populations was not statistically different. JEV genotype was not identified.
In Cambodia, another study from Kandal Province, field samples collected from chickens, dogs, ducks, and pigs had JEV seroprevalence rates of 1%, 35%, 12%, and 31%, respectively (Landreyt et al., 2020). JEV seroprevalence was similar for dogs and pigs, and JEV force of infection, reported as the rate of JEV infection in a susceptible population per unit of body surface area (BSA) in chickens, dogs, ducks, and pigs was 0.003, 0.01, 0.03, and 0.09, respectively. Data indicate that ducks and pigs are more likely to be infected with JEV than chickens and dogs.
JEV is known to circulate throughout Cambodia, and low-densities of domestic pig populations suggests that JEV has a complex multi-host system. Although JEV seroprevalence in domestic chickens was low compared to other species, another field JEV serological study conducted in three Cambodian provinces involving domestic chickens (Gallus gallus domesticus) and domestic ducks (Anas platyrhynchos domesticus) showed that 43.5% of chickens and 42.3% of ducks were positive for JEV antibodies (Auerswald et al., 2020). Although these studies suggest that poultry (e.g. chickens and ducks) may be JEV maintenance hosts, research has not been published that fully explains poultry’s natural role in JEV maintenance (Landreyt et al., 2022). This lack of understanding may be a significant knowledge gap (Walsh et al., 2022).
Globally, more than 90 bird species are recognized as JEV reservoir and amplifying hosts (Yap et al., 2019).
Bats
Additional evidence suggests that bats may also play a role in JEV maintenance, including dispersal and overwintering.
In China, JEV genotype III has been isolated from a caught Leschenault’s rousette (Rousettus leschenaultii) and a caught Tube-nosed bat (Murina aurata) in Yunnan Province, China (Wang et al., 2009). JEV genotype III has also been isolated from a caught Rickett’s big-footed bat (Myotis ricketti) in Huizhou, Guangdong Province; a Common bent-wing bat (Miniopterus schreibersii) in Haikou, Hainan Province; and a Common bent-wing bat and an Intermediate horseshoe bat (Rhinolophus affinis) in Yueyang, Hunan Province (Liu et al., 2013).
In Indonesia, JEV of unspecified genotype has been detected in caught Greater short-nosed fruit bats (Cynopterus sphinx), Lesser long-tongued fruit bats (Macroglossus minimus), Lesser short-nosed fruit bats (Cynopterus brachyotis), and Spotted-winged fruit bats (Balionycteris maculata) in West Kalimantan Province in coastal, forest, and residential areas (Diptyanusa et al., 2021). And JEV of unspecified genotype has been isolated in caught bats in the Cynopterus (Fruit bats), Eonycteris (Dawn fruit bats), Hipposideros (Roundleaf bats), Kerivoula (Evening bats), Macroglossus (Long-tongued Nectar bats), Pipistrellus (Evening bats), Rousettus (Rousette bats), Scotophilus (Evening bats), and Thoopterus (Fruit bats) genera (Diptyanusa et al., 2022). Bats positive for JEV were caught in Bali Province, East Java Province, East Nusa Tenggara Province, North Sulawesi Province, Riau Province, West Java Province, West Kalimantan Province, and West Sumatra Province (in forest, coastal, and urban areas).
In Australia, Black flying foxes (Pteropus alecto) may also have a role in JEV dispersal (van den Hurk et al., 2009). Captured bats that were either experimentally inoculated with JEV (N = 5) or exposed to JEV infected Culex annulirostris mosquitoes (N = 10) developed IgG antibodies (5/5, 6/10). Although low-level JEV viremia was detected in only one of the experimentally inoculated flying foxes and in none of the bats that were exposed to infected Culex mosquitoes, at least one flying fox from each group was able to infect recipient Culex mosquitoes. Black flying foxes inhabit a large range throughout Australia, Indonesia, and New Guinea, and they are migratory and typically roost in large colonies with other flying fox species.
The JEV cycle between bats and mosquitoes may involve several routes of infection (Diptyanusa et al., 2022). Insectivorous bats may consume JEV infected mosquito species. Additionally, mosquitoes may be infected while feeding on partially eaten fruit discarded by JEV infected frugivorous bats. These theories suggest that JEV infected feeding mosquito species may not be the only mechanism of JEV infection in bats.

Flying foxes in Australia. Source: Cecila Sanchez, EcoHealth Alliance.
JEV has not been detected in North America. However, information about North American bats and their spatial ranges are available through these sources: Bats of Canada, Bats of Mexico, Bats of the United States.
Marsupials
Experimental inoculations of Australian marsupials including the Eastern grey kangaroo (Macropus giganteus), Tammar wallaby (Notamacropus eugenii), and Agile wallaby (Notamacropus agilis) have resulted in very low or undetectable levels of JEV viremia (Mackenzie et al.,2022). However, Brush-tailed possums (Trichosurus vulpecula) inoculated with JEV did develop viremia sufficient for infectious JEV to be recoverable over several days, which suggests that this species may be a potential JEV maintenance host.
JEV amplifying hosts
Pigs
Wild boars / feral pigs (Sus scrofa) and domestic pigs (Sus scrofa domesticus) play important roles in JEV epidemiology, including maintenance, amplification, and as the source of infections transmitted to humans (Komiya et al., 2019 and Ladreyt et al., 2019).
JEV seroprevalence in populations of domestic pigs (of mixed ages) between 1966 and 2016 in 14 countries and Hong Kong suggests that JEV infections have been widespread (Ladreyt et al., 2019), with the highest seroprevalence in Laos (75%), Thailand (74%), Australia/Torres Straight (70%), India (67%), Cambodia (64% – 66%), Hong Kong (60%), and Vietnam (60%). Countries with lower seroprevalence were Malaysia (44%), Indonesia (40%), Taiwan (37%), Myanmar (33%), Sri Lanka (33%), Nepal (17%), and Ishigaki Island, Japan (3.1%).
During the same period, high JEV seroprevalence was found among wild boar and/or feral pigs in Singapore (100%), Japan (83%), Australia/Torres Straight (79%), South Korea (66%), and Iriomote Island, Japan (44%).
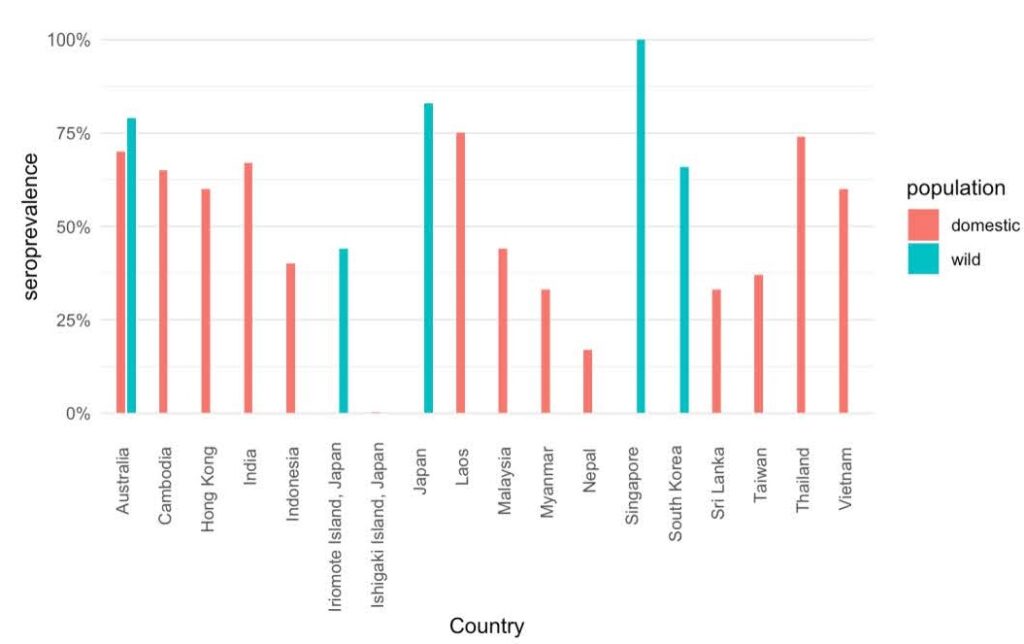
JEV seroprevalence in domestic and wild pigs recorded between 1966 – 2016 in identified countries. Figure adapted from Ladreyt et al., 2019. Credit: Éric Marty, CEID, University of Georgia.
In Bangladesh, a 2009 survey of 11,364 domestic pigs in Nawabganj District, Naogaon District, and Rajshahi District estimated that 30% of the population had JEV IgG antibodies (Khan et al., 2014). The authors also suggested that JEV infects 20% of the susceptible pig population each year, and estimated the reproductive number (R0) to be 1.2.
Birds
Little egrets (Egretta garzetta), Black-crowned night herons (Nycticorax nycticorax), and other wading birds are highly susceptible to JEV infection and produce a viremia sufficient to infect feeding mosquitoes (Le Flohic et al., 2013). However, the role that birds have in amplifying JEV still needs considerable research (Hameed et al., 2021).
In South Korea, human JEV cases between 2011 and 2016 were positively correlated with the presence of herons and not pigs, particularly in cities (Bae et al., 2018). During this period, 9.6 million pigs were bred in South Korea, and approximately half were raised in Chungnam Province, Gyeonggi Province, and Jeonbuk Province. During the same period, just over 15,000 pigs were bred in the cities of Daegu, Daejeon, and Seoul. The human incidence of JEV infections was higher in cities than in provinces. In a corresponding analysis of 131 JEV human cases and 17 deaths in South Korea, human JEV cases were not correlated with the presence of pigs. Ardeid birds that frequently nested in Chungnam-Daejeon-Sejong Province, Gyeongbuk-Daegu Province, and Seoul-Gyeonggi-Incheon Province included Gray herons (Ardea cinerea), Great egrets (Ardea alba), and Little egrets (Egretta garzetta).
Some authorities have suggested that Ardeid and other bird species can act as JEV amplifying hosts, but additional research is needed to determine the relevant range of competent species.
In a landscape epidemiology study of JEV outbreaks in India from 2010 – 2020, natural wetlands such as rivers and fresh-water marshes that share landscapes with rain-fed agriculture production systems on which Ardeid birds, chickens, and pigs converge concluded that there is an association between chickens and JEV spillover into human populations (Walsh et al., 2022). Although some studies involving experimental JEV infection in chickens and ducks have been published that demonstrated JEV virema levels sufficient to infect feeding mosquitoes, additional research to understand the role that chickens, ducks, and other poultry have in JEV amplification is needed.
Mosquito vectors
Seventeen mosquito species are known to be JEV-competent vectors (Auerswald et al., 2021 and van den Eynde et al.,2022), exhibiting a range of feeding habits and distributions. Details and species distribution maps are provided in the table below.
JEV has also been detected in the field in over 20 mosquito species not proven to be competent JEV vectors (van den Eynde et al., 2022).
Mosquitoes typically prefer a single host species for blood-feeding; mosquito host preferences have been documented in numerous studies, including Hoyos et al. (2021). However, mosquitoes may shift their feeding to an alternate species if the preferred species is unavailable. Diptyanusa et al. (2021) reported that JEV-competent female Culex mosquitoes (C. tritaeniorhynchus, C. vishnui) may shift their feeding preferences from a preferred species (e.g. pigs) to another vertebrate when when the preferred species is not available.