Last Updated on February 18, 2025
This page describes:
- JEV in humans
- Epidemiological patterns
- Map of JEV genotypes by Country
- The 2022 Australian genotype IV outbreak and Clinical Swine Epidemiology
- Diagnostics
- Vaccination
JEV in Humans
Transmission

Humans are infected with Japanese encephalitis virus (JEV) when bitten by an infected mosquito such as Culex tritaeniorhynchus (Auerswald et al., 2021). Human JEV infections are not caused by the consumption of infected pork, and humans cannot be infected when in close contact with an infected animal such as a domestic pig. Humans do not directly spread JEV to other people. However, human JEV infections caused by contaminated blood donations have been reported (Cheng et al., 2018).
JEV has a 5–15 day incubation period in humans (CDC, 2022).
Symptoms
JEV is considered the most important cause of human viral encephalitis in all of Asia, with 86% to 95% of reported cases occurring in China and India (Muniaraj et al., 2019). Globally, an estimated 175,000 people annually are infected with JEV (Mulvey et al., 2021). The majority of human infections are either asymptomatic or result in mild discomfort. A small number of cases (0.1% – 1%) will develop encephalitis (Turtle et al., 2018). The current, estimated overall case fatality rate (CFR) of those who develop serve disease is 14% (Cheng et al., 2022).
Clinical symptoms of mild JEV infection include headache, fever, and vomiting (CDC, 2022).
Clinical manifestations of severe disease (Scheld et al., 2008, Turtle et al., 2018) include:
- Development of severe clinical symptoms
- Mental status changes
- Focal neurologic defects
- Generalized weakness
- Movement disorders
- Presentation of Parkinsonian syndrome symptoms
- Dull flat, mask-like facies with wide unblinking eyes
- Tremor
- Cogwheel rigidity
- Choreoathetoid movements of the fingers or toes
- Development of poliomyelitis-like acute flaccid paralysis
- Development of seizures, especially in children
- Twitching of a digit
- Eye deviation
- Irregular breathing
- Clinical signs of death
- Status epilepticus (5 minutes or more of continuous seizure)
- Brain hypoxia
- Increased intracranial pressure
- Brainstem herniation (shifting of brain tissue)
Thirty to 50% of patients who survive severe clinical disease will exhibit long-term psychiatric and neurologic effects (Amicizia et al., 2018).
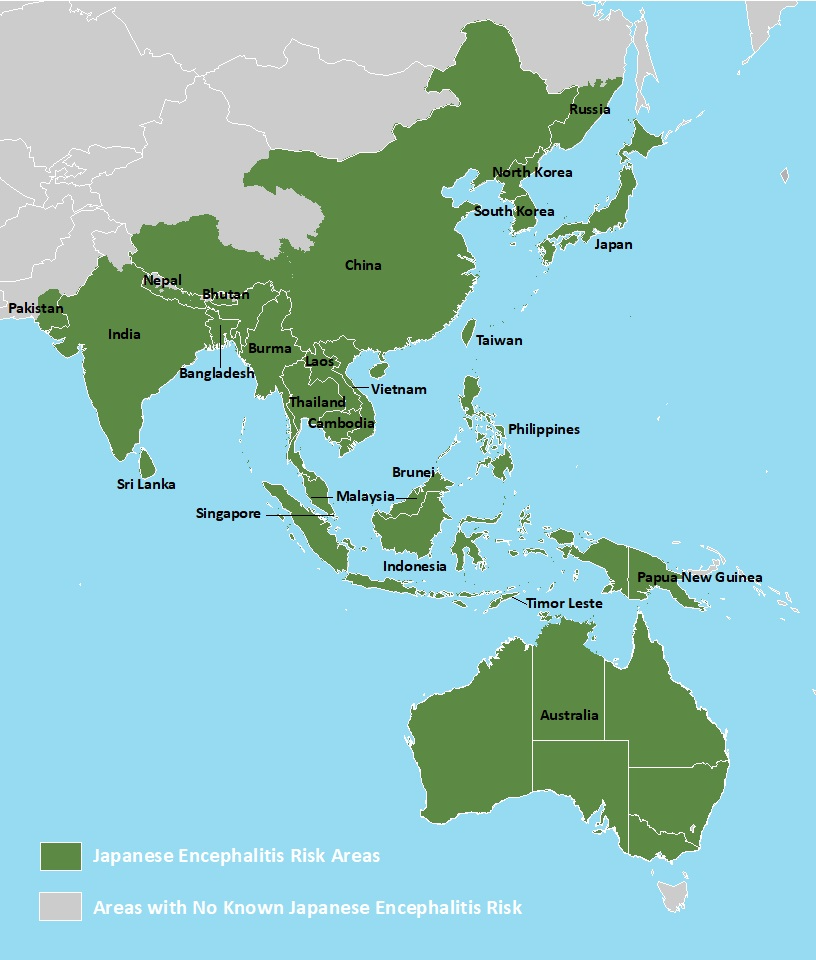
Spatial distribution
Human cases of Japanese encephalitis (JE) have been documented in 25 countries (CDC, 2022). In March 2016, one locally acquired JEV genotype III infection was identified in a 19-year old man in Angola during an outbreak of yellow fever (Simon-Loriere et al., 2017). And although human cases have not been documented in Europe, JEV genotype III was detected in birds and Culex pipiens mosquitoes in Tuscany, Italy (Ravanini et al., 2012 and Gao et al., 2019). In Italy, JEV strains from Culex mosquitoes were identical to JEV strains collected from bats in China.
Age distribution
In endemic countries, JEV is primarily a disease of children with many cases reported in individuals under the age of 15 (Wang et al., 2015, Henriksson et al., 2021, and Allen et al., 2023). During the 2021–2022 Australian outbreak of JEV, 76% of human cases were identified in people aged 40 or over. Such a difference in age distribution between endemic and previously non-endemic countries can be attributed to a lack of childhood exposure to the disease. Frequent, early exposure and childhood immunization provides adults with protection that is absent in immunologically naive populations (McGuinness et al., 2023).
Mortality by country
A recent modeling effort (conducted prior to the 2022 outbreak in Australia) attempted to quantify case fatality rates (CFR) by country based on a series of covariates thought to be correlated to JE mortality, including population growth rate, gross domestic product (GDP), and mortality rates in children under 5 (Cheng et. al., 2022). CFR was modeled for 20 countries with endemic JEV for four past periods and for 2019–2030. Countries with the highest predicted CFR for the period 2019–2030 are Bangladesh (28%), Guam (24%), Pakistan (23%), the Philippines (22%), and Vietnam (22%). Those with the lowest predicted CFR are Cambodia (7%), Indonesia (7%), Myanmar (6%), China (5%), and Thailand (4%). These predictions do not include Australia, since JEV was not endemic there at the time of the study.
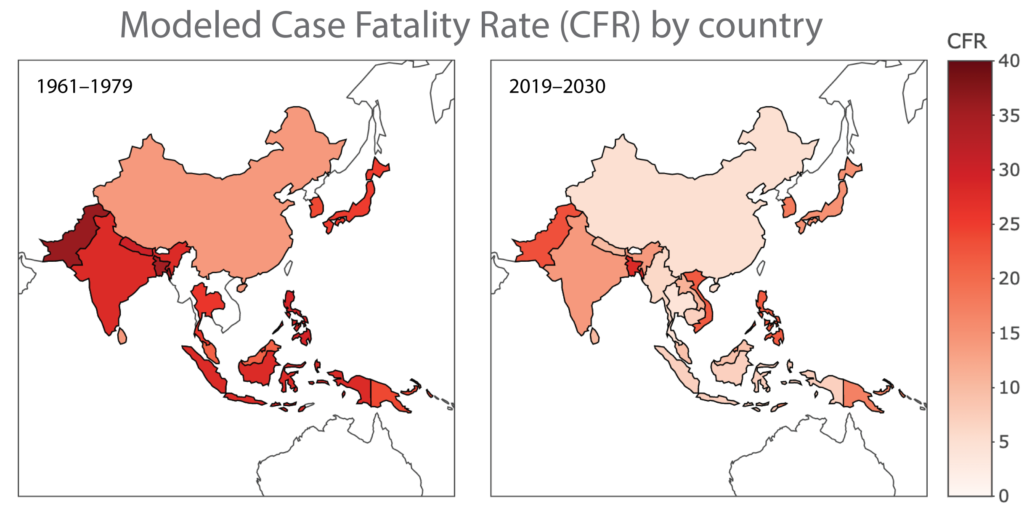
Notably, the documented CFR for Australian for the period from January 1, 2021 through February 2023 was 15.2% (McGuinness et al., 2023).
Globally, modeled CFR dropped from an estimated 26% for 1961–1979 to ~14% for the period 2000–2018 (Cheng et. al., 2022).
CFR has been correlated with either national or subnational JEV vaccination programs. As of June 2020 vaccination programs exist in Australia (Islands in the Torres Strait), Cambodia, China, India (~50% of all districts), Indonesia (Bali), Japan, Laos, Myanmar, Nepal, South Korea, Sri Lanka, the Philippines, and Vietnam (Vannice et al., 2021). As a result of the 2022 Australian JE outbreak, JEV vaccination programs have been implemented in the northernmost section of the Northern Territory including the Tiwi Islands and in the Murray-Darling River Basin (McGuinness et al., 2023).
Risk factors
Due to its close association with rice irrigation and pig rearing, JE is considered a rural disease, though sporadic urban cases have been reported (Francesco et al., 2018). Risk factors for JE in humans include:
- Being a child below age 15.
- Living in rural or peri-urban regions with close proximity to pigs and rice fields.
- Being unvaccinated for JEV.
- Absence of prior flavivirus infection (Libraty et. al. 2002).
Epidemiological Patterns
Where JEV occurs, two distinct epidemiological patterns are recognized: endemic and epidemic (Wang et al., 2022).
Epidemic patterns are characterized by seasonality and occasional outbreaks, with cases peaking in summer and fall. Epidemic patterns are typically seen in countries in the northern range of JEV occurrence with a more temperate climate (CDC, 2022), including Bangladesh, Bhutan, China, Taiwan, Japan, South Korea, North Korea, Nepal, Pakistan, Russia, and the northern regions of Vietnam, India and Thailand (Wang et al., 2022).
Endemic patterns characterized by sporadic outbreaks occurring throughout the year are more likely to be found in countries in southern Asia and Oceania, such as Australia, Burma, Brunei Darussalam, Cambodia, Indonesia, Laos, Malaysia, Papua New Guinea, Philippines, Singapore, Sri Lanka, Timor-Leste, and the southern regions of Vietnam, Thailand, and India (Wang et al., 2022).
JEV Genotypes
JEV has one serotype and 5 genotypes (Rajaiah et al., 2022). Clinically, all JEV genotypes have been associated with human infections and encephalitis. However, no differences in human disease severity between genotypes have been reported (Mackenzie et al., 2022).
JEV is believed to have originated around 1,000 CE in the Malaysia/Indonesia region from an African ancestral virus (Solomon et al., 2003, Xu et al., 2023). Phylogenetic evidence suggests that JEV genotype V was the first to evolve from a most recent common ancestor that additionally diverged into Murray Valley encephalitis (Schuh et al., 2013, Xu et al., 2023). Subsequent temporal evolution of JEV genotypes I–IV followed this specific order: genotype III, genotype II, genotype I, and finally genotype IV (Xu et al., 2023). JEV genotype IV is estimated to have emerged 122 years ago and has undergone the fastest evolution (Xu et al., 2023).
JEV spread from the Malaysia/Indonesian region onto the Indochinese Peninsula, and later onto the Asian continent (Solomon et al., 2003, Xu et al., 2023). Since the 1990s, JEV genotype I has displaced JEV genotype III and is now the predominate genoytpe in Asia (Xu et al., 2023).
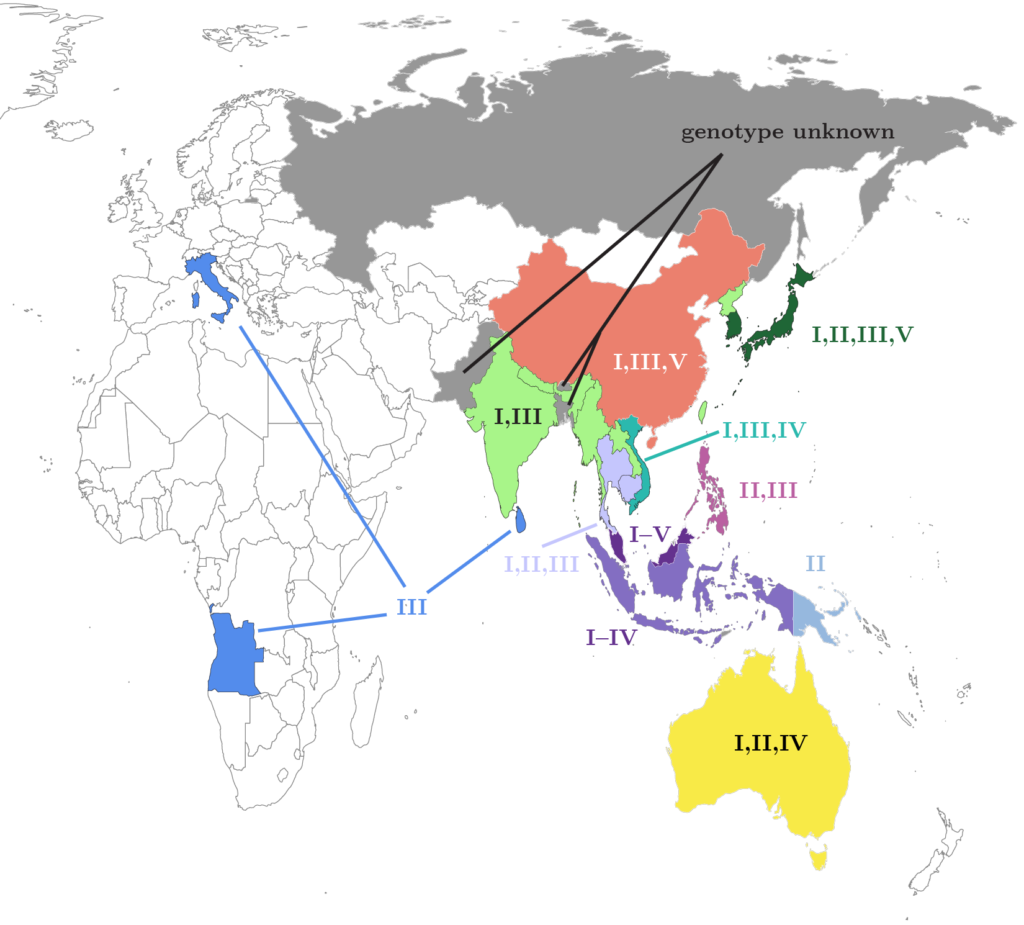
Spread of JEV genotype IV
Phylogetic evidence suggests that JEV genotype IV originated in a geographic area that includes Vietnam and Indonesia, and began to spread southward through the Indonesian archipelago and reached Oceania between 2005 and 2020 (Xu et al., 2023). JEV genotype IV was first isolated from Culex tritaeniorhynchus, Culex vishnui, or mixed pools of mosquitoes trapped in 1980 and 1981 on the Indonesian islands of Bali, Java, and Flores (Solomon et al., 2003, Mackenzie et al., 2022). A retrospective analysis of a human JEV isolate (VN113) collected in Vietnam in 1979 identified it as JEV genotype IV (Mackenzie et al., 2022). However, case travel history and clinical information is unknown.
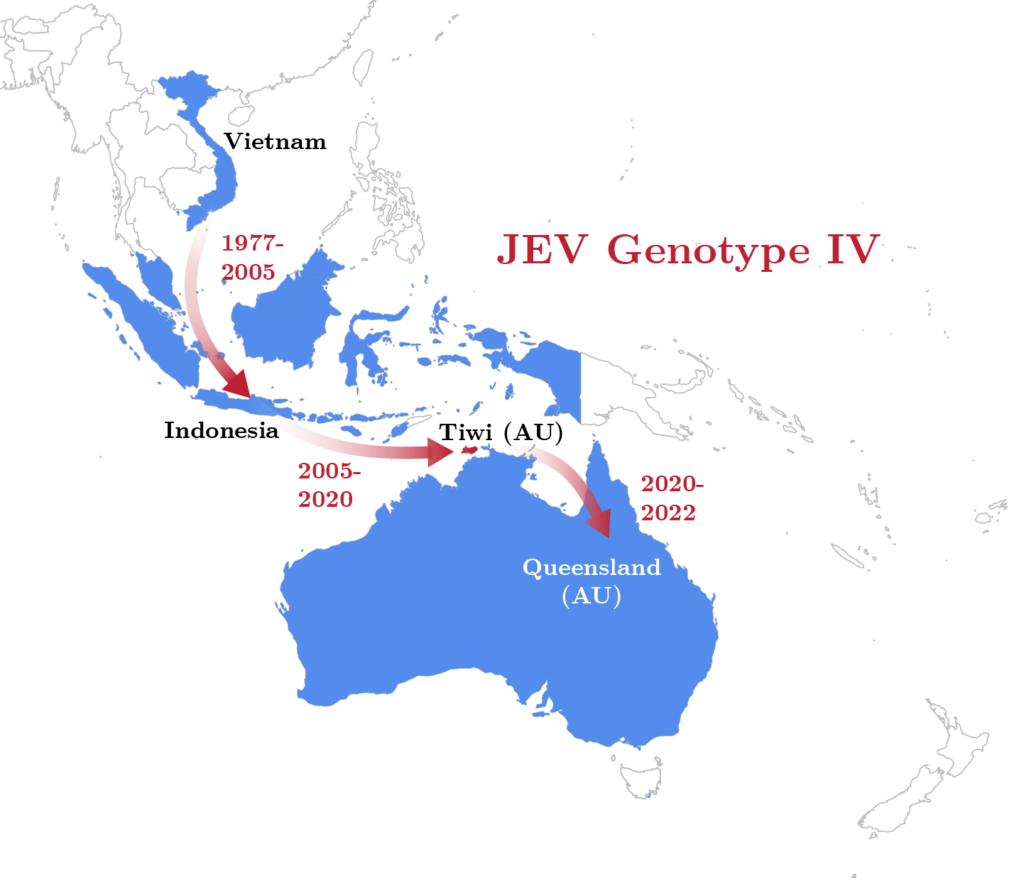
In 2017, two JEV genotype IV isolates collected from pigs on the island of Bali, Indonesia were determined to be similar to three JEV genotype IV isolates collected from mosquitoes on the islands of Flores and Java in 1981 (Kuwata et al., 2020). In 2019, JEV genotype IV was isolated from Culex vishnui mosquitoes on Bali and was found to be 99% similar to the two JEV genotype IV isolates collected from pigs on Bali in 2017 (Faizah et al., 2021, Mackenzie et al., 2022). In November 2019, a 59-year-old unvaccinated Australian who contracted JEV genotype IV while on Bali died in a Brisbane hospital after spending more than three months in an ICU (Pyke et al., 2020, Mackenzie et al., 2022). The isolate from this patient was 99.23% similar to the two swine JEV genotype IV isolates collected in 2017 on Bali and 95.38% similar to the JEV genotype IV isolate collected on Flores in 1981.
Before the February 2021 death of a 45-year old living in the Tiwi Islands, Australia, JEV genotype IV had not been isolated east of Flores. Flores lies ~1,100 km north-west of the Tiwi Islands. The introduction of JEV genotype IV into Australia may have occurred via migratory birds that infected resident feeding mosquito species. Alternately, its introduction may have been the result of infected mosquito species that were wind-blown or transported on aircraft or ocean shipping from Indonesia (Waller et al., 2022). In 2020, Culex tritaeniorhynchus mosquitoes were documented for the first time in Australia in the vicinity of Darwin and Katherine. A mosquito specimen collected in Australia had a 99.7% nucleotide similarity with a Culex tritaeniorhynchus mosquito collected in 1999 in Dili, Timor-Leste (Lessard et al., 2021). Darwin and Dili are separated by the Timor Sea and are ~640 km apart. The introduction of Culex tritaeniorhynchus from Timor-Leste into Australia’s Northern Territory may have occurred from wind dispersion or transport by aircraft or shipping.
Spread of genotypes I and II from Papua New Guinea to Australia
Papua New Guinea (PNG) was the proximate origin of JEV genotypes I and II that spread into Australia. Human JEV seroepidemiogical studies conducted in PNG’s Western Province, Gulf Province, and Southern Highlands Province between 1989 and 1998 determined that unreported JEV antibody positivity rates appeared to be increasing, and the spatial distribution of JEV was moving eastward (Mackenzie et al., 2002). Seroepidemiogical studies in swine conducted in the Western Province between 1995 and 1996 concluded that 50% of surveyed pigs had JEV antibodies. The first four clinical cases of JEV in humans and first two human fatalities were reported in 1997 and 1998 in a Kiunga hospital located in the Western Province (Mackenzie et al., 2002).
Australia saw its first cases of JEV genotype II in humans with the 1995 outbreak in the Torres Strait island of Badu (three cases, two fatalities), and the 1998 cases on Badu and on mainland Australia near the mouth of the Mitchell River in Queensland’s Cape York Peninsula. These outbreaks may have originated in infected birds, or possibly infected mosquitoes wind-blown from Papua New Guinea (Mackenzie et al., 2002, Waller et al., 2022). One JEV isolate from Culex sitiens mosquitoes collected in PNG in 1997 at the Lake Murray Patrol Post was identical in the nucleotide region of the prM gene to three isolates from the 1995 Badu Island outbreak (Johansen et al., 2000). Two isolates from Culex sitiens mosquitoes collected in PNG in 1998 at Balimo and Abam were identical in the the same region to one 1998 isolate from Badu.
Indonesia and/or Malaysia were the ultimate source of the JEV genotype II that reached Australia via PNG. The 1998 PNG and Badu JEV genotype II isolates were 99.5% similar, and both were 95% similar to a genotype II isolate from a C. tritaeniorhynchus mosquito collected in 1979 on Java, Indonesia, and to another isolate from a C. tritaeniorhynchus collected in Selangor State, Malaysia in 1994 (Schuh et al., 2013, Tsuchie et al., 1997).
In early 2000, JEV genotype I was identified in domestic pigs on the Torres Strait islands of Badu and Moa Island, near Mainland Australia, as well as in Culex gelidus mosquitoes on Badu. It was also identified in Culex annulirostris mosquitoes on Saibai Islan less than 10 km from the southern shoreline of Papua New Guinea (Pyke et al., 2001). Phylogenetic analysis determined that the Torres Strait isolates (TS00 and TS4152) had identical prM gene and E gene sequences. These isolates had a 97.5% prM gene similarity to a genotype I isolate from Thailand (Th2372, 1972), a 97.3% E gene nucleotide similarity to a genotype I isolate from Thailand (B-2239, 1984), and a 97.3% NS5-3′-UTR similarity to a genotype I isolate from Korea (K94P05, 1994). Sentinel pig herds and sentinel mosquitoes located on Badu and Moa confirmed the presence of JEV each year between 2001 and 2005.
The first confirmation of JEV on Australia’s mainland came in 2004, from a JEV genotype I isolate collected from a pool of Culex sitiens mosquitoes in Bamaga, 40 km south of the tip of the Cape York Peninsula (van den Hurk et al., 2006). The isolate was 99.6% and 99.8% similar to Torres Strait isolate TS00 in the C-prM gene and E genes, respectively. JEV continued to be identified in Torres Strait swine and horses in 2010, 2012, 2013, 2014, 2016, 2017, and 2019 (Mackenzie et al., 2022), and unconfirmed JEV was reported in pigs and horses in the Northern Peninsula Area (NPA) in 2020 and 2021.
The 2022 Australian JEV Genotype IV Outbreak
A retrospective analysis in 2021 of serum samples collected from 168 feral pigs in the Northern Peninsula Area identified one animal with an exposure to JEV (Animal Health Surveillance Quarterly, Volume 26, Issue 4). A PCR test for JEV was negative, and active JEV viremia was not occurring when the serum sample was collected. The retrospective serum analyses were performed because of a locally acquired human JEV infection and death the first quarter of 2021 in the Tiwi Islands in the Timor Sea north of Darwin. This case was later identified as a sentinel case of the 2022 Australian JEV genotype IV outbreak (Waller et al., 2022).
In early January 2022, commercial pig operations in eastern and southern Australia began reporting delayed farrowing in sows, aborted fetuses, and infertile boars (Dr. Mark Schipp, Australian Chief Veterinary Officer). Clinical signs also included mummified fetuses, stillbirths, piglet physical malformations such has deformed heads and limbs, tremors, ascites, and piglets born without brains.

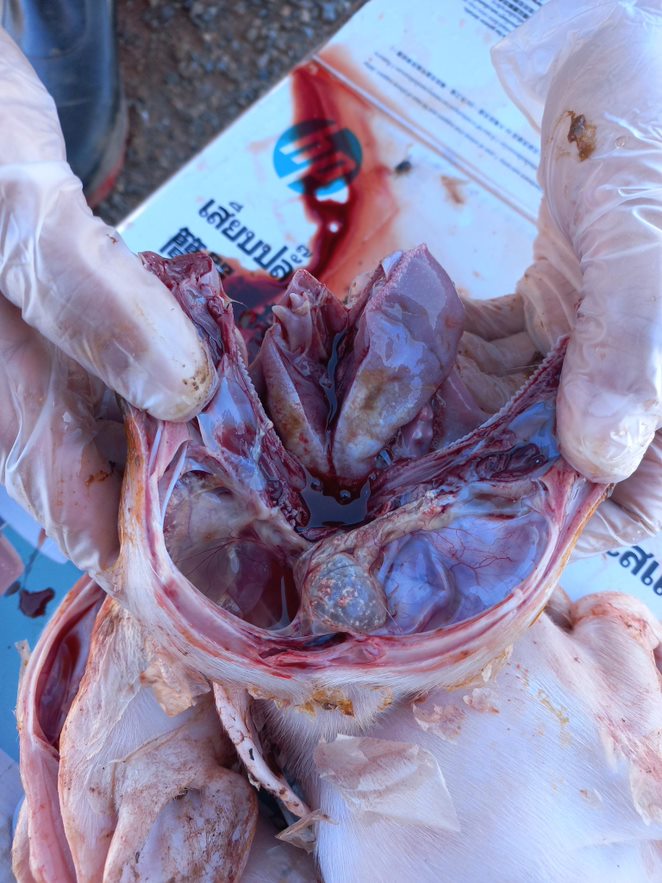
Piglets born from JEV infected sows. Images courtesy of Drs. Bernie Gleeson and Kirsty Richards, SunPork Farms, Australia.
On February 25, 2022, JEV genotype IV was confirmed in commercial piggeries in Queensland and New South Wales, and the next day JEV genotype IV isolates were identified in infected piggeries in Victoria. JEV genotype IV was later identified in pig herds in South Australia (Yakob et al., 2023, Dr. Mark Schipp, Australian Chief Veterinary Officer). On March 3, the first human case of JEV genotype IV was identified in Queensland (WHO, 28 April 2022), and on March 4, the JEV outbreak was declared a Communicable Disease Incident of National Significance by the Australian Government Department of Health and Aged Care.
Spread of Genotype IV from Bali, Indonesia
JEV genotype IV was identified as the causative agent of the JE outbreak through phylogentic analysis of the envelope (E) gene (Howard-Jones et al., 2022). The origin of the 2021 Tiwi Islands human JEV isolate and 2022 isolate collected from aborted swine at a Queensland piggery is believed to be Bali, Indonesia (Sikazwe et al., 2022). The Tiwi Island and Queensland piggery isolates have a 99.8% nucleotide similarity. The Tiwi Island isolate has a 96.8% nucleotide similarity to JEV genotype IV isolates collected from pigs in 2017 on Bali and a 96.7% nucleotide similarity to the 2019 JEV genotype IV human isolate from Bali that resulted in the death of an Australian traveler. JEV genotype IV has not been isolated in Papua New Guinea, West Papua, Indonesia, nor in Timor-Leste (Sikazwe et al., 2022).
The JEV genotype IV outbreak affected more than 80 piggeries, impacted ~60% of the Australian pork industry, and had an economic cost of US $215,000–$250,000 per 1000 sows (Dr. Kirsty Richards, SunPork Farms Veterinarian). Through February 2023, the outbreak infected 46 people and killed 7 (CFR of 15.2%) (McGuinness et al., 2023). 76% of all cases were in people aged 40 or older, and 31% percent in women. 85% percent of confirmed and probable cases occurred in New South Wales (14 cases), South Australia (10), and Victoria (15). 71% percent of deaths occurred in New South Wales (2), South Australia (2), and Victoria (1).
In descending order, an analysis of human JEV cases determined that living in or visiting a rural area was the predominate factor that contributed to an infection, followed by camping, participating in a water sport such as boating, working on a farm, or working outside (Veliz et al., 2025). Associations with piggeries (e.g., abattoir, rendering plant, animal/feed transport related to pigs) were not significant factors.
Diagnostics
The World Health Organization recommends testing suspected JEV cases through identification of a JEV-specific IgM antibody in serum or cerebrospinal fluid (WHO, 2019). Testing with cerebrospinal fluid is the preferred method, as this helps reduce false-positive rates from vaccines or previous infection. Most commonly, an ELISA kit test is used to diagnose JEV by detecting the IgM antibody, which can be identified within 7 days from the onset of symptoms. Commercially available ELISA kits can be used for JEV detection in both humans and pigs. With additional testing, it is possible to distinguish between antibodies produced in response to infection and those in response to vaccines. Other studies site the benefit of detecting the whole virus rather than the antibody through PCR, Plaque Reduction Neutralization Test, or Virus Isolation because JEV can be identified from the first day of infection, whereas antibodies take several more days for the body to produce (Roberts et al., 2020).
Vaccination
The first generation of JEV vaccines were developed in Japan in the early 1930s (Satchidadanandam, 2020). Approved for human use in 1954, these vaccines were largely responsible for the decreased rates of JEV incidence rates seen in Japan and Taiwan by the 1950s. Derived from mouse brains (MB-JEV), these inactivated vaccines were highly efficacious. However, the multiple dose regime and a rare side effect of disseminated encephalomyelitis led to discontinuation of its use in Japan in 2011. Other south Asian countries continue its use, and since then several other vaccines have become available in the market.
Currently, there are four vaccine types: mouse-brain derived (inactivated), Vero cell derived (inactivated), live recombinant and live attenuated (Furuya-Kanamori et. al. 2022). The live recombinant vaccine is the only one that is a single dose and requires no boosters. However, caution must be taken as it is contraindicated for immunocompromised individuals and pregnant women. Another recommendation is not to administer other live vaccines at least one month before or after receiving the live attenuated JEV vaccine. All other vaccine types require at least two doses and boosters administered every 1-2 years. Inactivated vaccines are recommended for those who are immunocompromised or for pregnant women, as these aren’t associated with the risks of live-attenuated vaccines (Furuya-Kanamori et. al. 2022).
Failure to complete the full immunization schedule of the multi-dose JEV vaccine is a common occurrence in rural areas where access to medical services is sparse (Zheng et. al. 2012). However, at least one vaccine does provide some immune response which, along with exposure to JEV infection, is thought to provide low-level protective immunity. Researchers believe this may explain why JEV infection rates are high in some regions with vaccine programs but mortality rates are disproportionately low compared to regions without vaccine programs (Zheng et. al. 2012).
Overall, the severity of JEV has declined throughout the decades with the implementation of vaccine and vector control programs, with some estimates placing CFR as low as 14% compared to 26-30% in the 1960s (Cheng et. al. 2022).
Additional information about commercially available JEV vaccines is available through UNICEF.